In recent years, the landscape of cell line engineering has undergone a profound transformation.
Researchers who once relied on traditional gene knockout methods are now turning to more efficient tools for creating stable knock out cell lines to accelerate discovery and reduce development cycles.
At the heart of this revolution lies a breakthrough that has moved from buzzword to lab bench essential: CRISPR gene editing, which continues to expand what’s possible in genetic research.
The pace of biomedical innovation has never been faster, and with it comes a growing demand for precision and control in genetic research. Whether in oncology, immunotherapy, or vaccine development, scientists need more than just a model—they need cell systems that behave predictably under complex experimental conditions.
This is where engineered models such as a knock out cell line come into play. By selectively disabling genes in a stable, traceable way, these lines give researchers the clarity they need to study pathways, validate targets, and screen new therapeutics with minimal background noise.
Traditional gene manipulation techniques often lack the specificity or scalability required in today’s research pipelines. As experimental targets become more nuanced—such as epigenetic regulation or multi-gene pathways—researchers can no longer rely on generalized models. Instead, they’re turning to gene-edited cell lines that replicate very specific genetic profiles, enabling more meaningful insights and better translational outcomes.
Beyond scientific rigor, there’s also a business case. In drug discovery, delays in identifying the right cell model can lead to wasted resources and longer time-to-market. That’s why many research institutions now prioritize streamlined access to validated, high-fidelity cell lines as part of their early R&D investment.
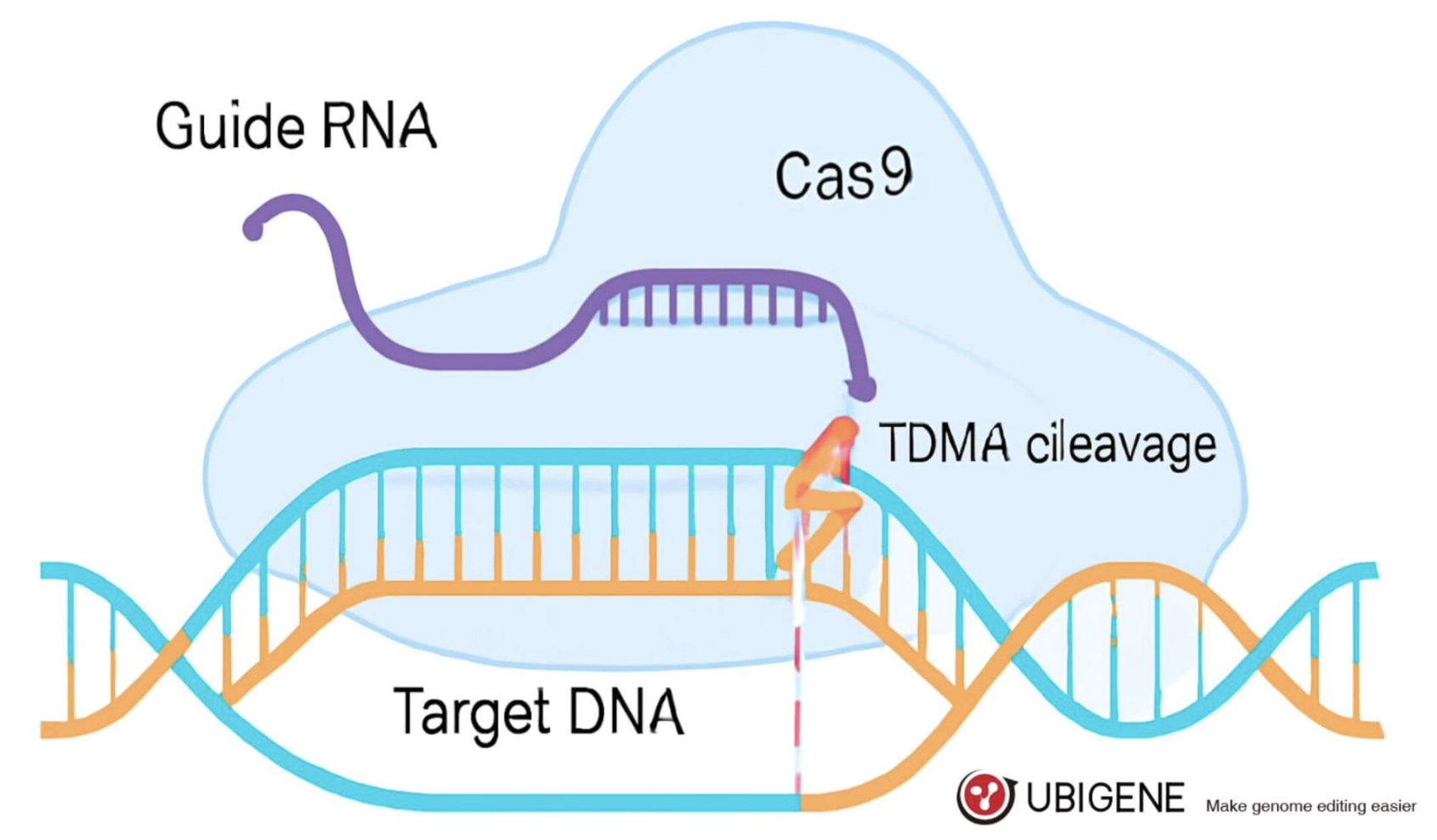
The journey of gene editing didn’t start with CRISPR—it’s the result of decades of incremental innovation. Earlier tools like Zinc Finger Nucleases (ZFNs) and Transcription Activator-Like Effector Nucleases (TALENs) laid the groundwork for programmable gene targeting. However, these methods were labor-intensive, expensive, and limited in precision.
CRISPR-Cas9 changed that.
Unlike its predecessors, CRISPR offers a simple, cost-effective, and highly scalable way to edit genes with near-surgical precision. With just a custom RNA guide and Cas9 enzyme, scientists can target almost any gene in the genome. This versatility has turned CRISPR into the default choice for generating knockout cell lines across laboratories worldwide.
Moreover, CRISPR’s open-access nature helped accelerate global adoption. As protocols became more refined and delivery systems improved—such as electroporation or lentiviral vectors—what once took months could now be done in a matter of weeks, with higher reliability and reproducibility.
This shift isn’t just technological—it’s strategic. By enabling faster, cheaper, and more precise gene knockouts, CRISPR has expanded the types of questions researchers can ask, and the speed with which they can answer them.
Not all knockout cell lines are created equal. For researchers aiming to derive accurate, reproducible results, quality isn’t optional—it’s foundational.
First, precision in gene targeting is key. A reliable knockout line should exhibit complete disruption of the target gene without off-target effects.
Second, clonal stability matters. A high-quality line maintains its genetic and phenotypic integrity through multiple passages.
Third, thorough validation is non-negotiable. Best practices include Sanger sequencing, Western blotting to confirm protein absence, and functional assays to demonstrate the gene’s loss-of-function impact.
Researchers are increasingly choosing to work with specialized services that provide pre-validated knock out cell lines, saving both time and risk in early-phase experiments.
The utility of knockout cell lines spans virtually every corner of the biotech and pharmaceutical industries. These engineered models are no longer confined to academic research—they’re now critical tools in applied settings, from early-stage discovery to preclinical validation.
In antibody development, knockout cell lines are used to eliminate endogenous gene expression that could interfere with target binding.
In oncology, CRISPR knockout lines are unlocking new therapeutic targets. By disabling genes involved in tumor proliferation or immune evasion, researchers are uncovering novel intervention points.
Beyond these areas, knockout lines support rare disease modeling, gene-drug interaction validation, and predictive toxicology screening.
If you’re exploring high-throughput applications, now is the time to explore trusted CRISPR gene editing services that simplify execution and enhance reproducibility.
In the early stages of drug discovery, knockout cell lines serve as essential validation tools. By creating models that lack specific disease-related genes, researchers can observe how candidate drugs behave in altered cellular environments. This approach helps de-risk the development pipeline by eliminating compounds with unintended interactions or low specificity, saving both time and investment.
As CRISPR technology matures, the focus is shifting from feasibility to optimization.
AI-powered tools assist in designing highly effective guide RNAs, reducing off-target risks.
Automation, through robotic systems, enables high-throughput cloning, screening, and validation—boosting both speed and consistency.
Integrated platforms now offer end-to-end CRISPR services, streamlining everything from gRNA design to clonal verification. For biotech firms and research institutions, this translates to reduced labor costs, higher throughput, and better regulatory documentation.
Emerging platforms also incorporate AI for post-editing analysis—such as predicting protein structure changes or pathway disruptions based on edited sequences. Combined with cloud-based systems for data storage and sharing, these tools empower global teams to collaborate seamlessly on large-scale knockout screening projects.
As research grows more complex, so does the need for precision-engineered cell lines. The best partners offer not just CRISPR tools, but validated, scalable solutions—backed by transparency, support, and speed.
Whether you’re building knockout models for discovery or preclinical use, partnering with a specialist can dramatically reduce risk and improve outcomes.
Now is a great time to learn more about knock out solutions that are lab-validated and ready to integrate into your next research breakthrough.
